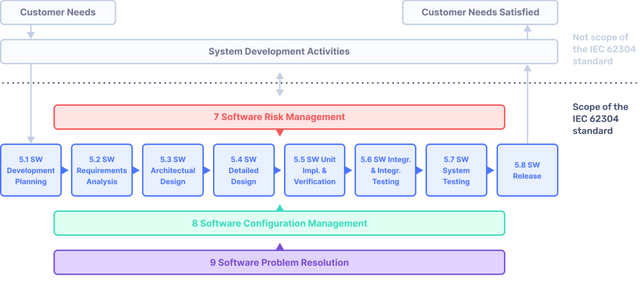
IEC 62304: Medical Device Software LifeCycle Processes | by Dr Stephen Odaibo | The Blog of RETINA-AI Health, Inc. | Medium
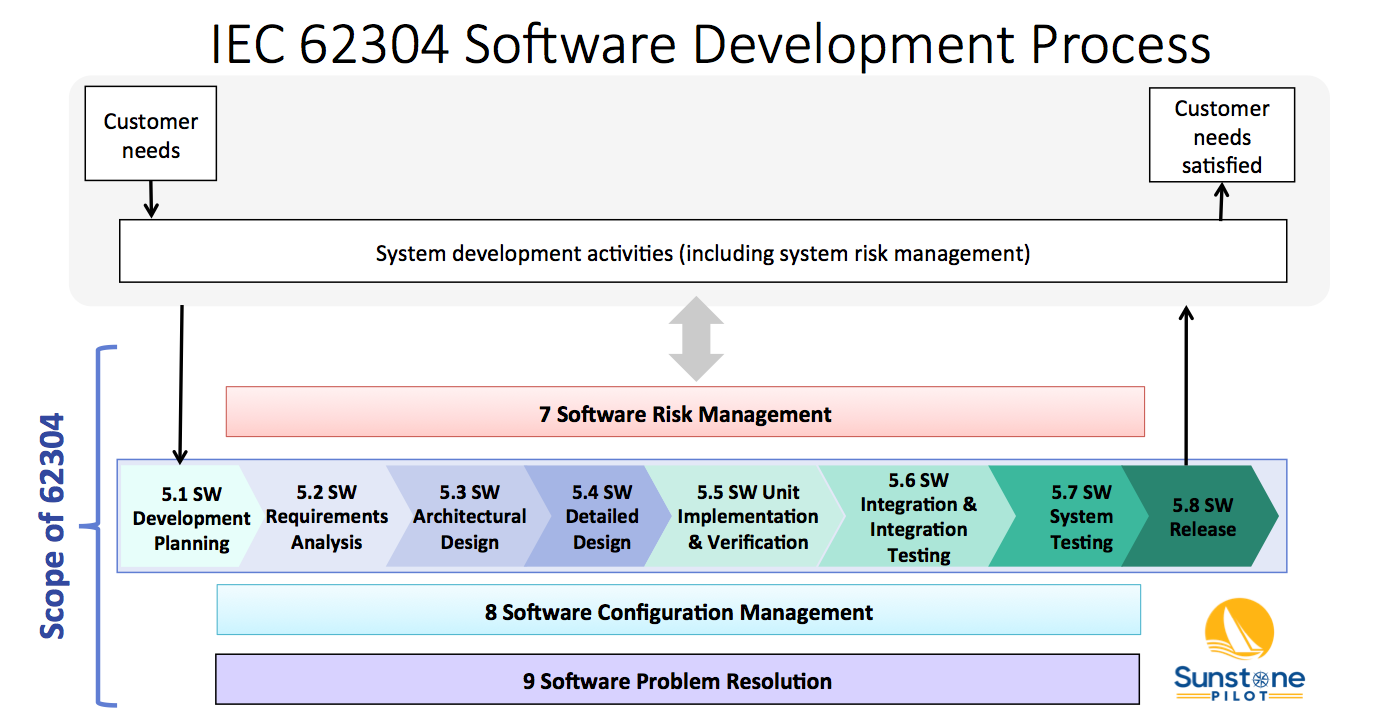
IEC 62304: Medical Device Software LifeCycle Processes | by Dr Stephen Odaibo | The Blog of RETINA-AI Health, Inc. | Medium

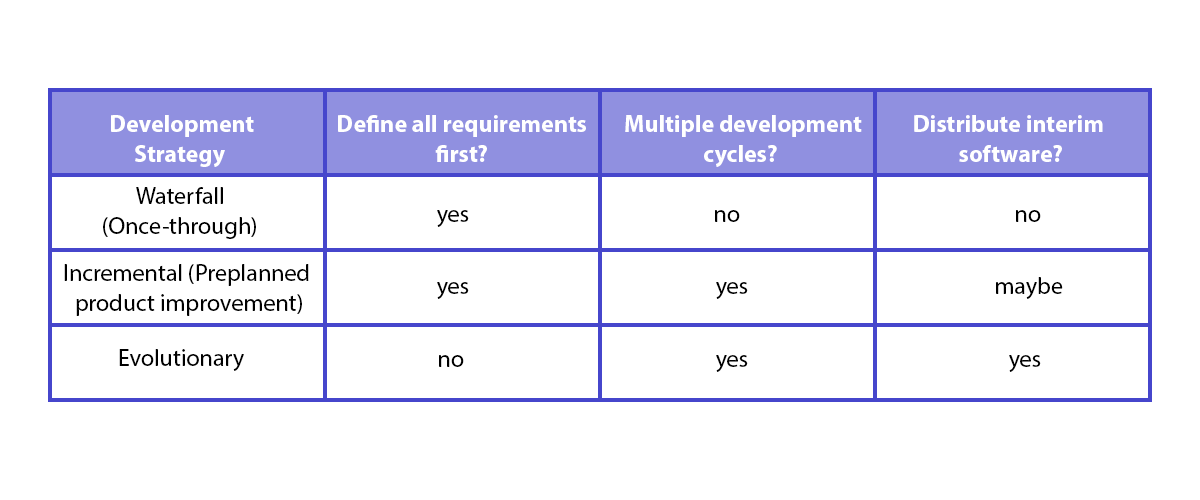
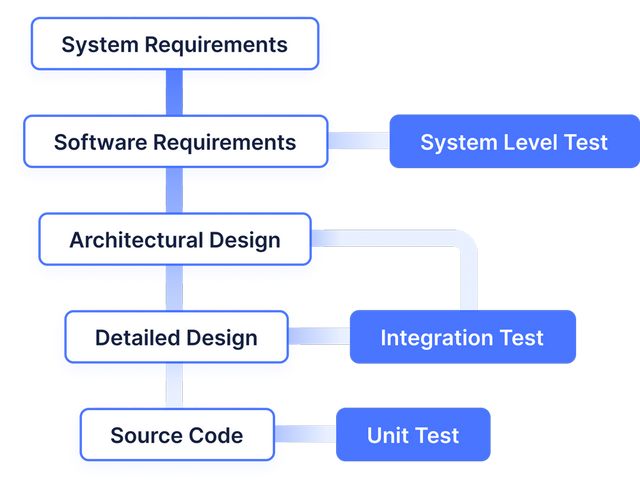
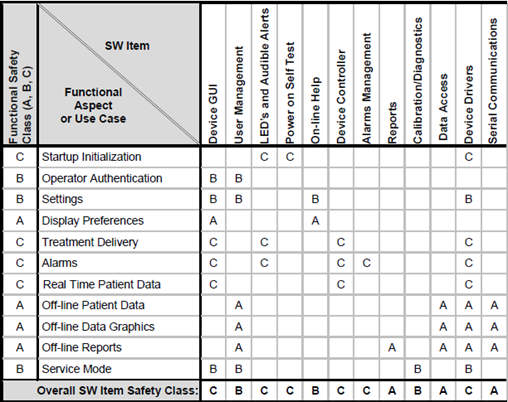
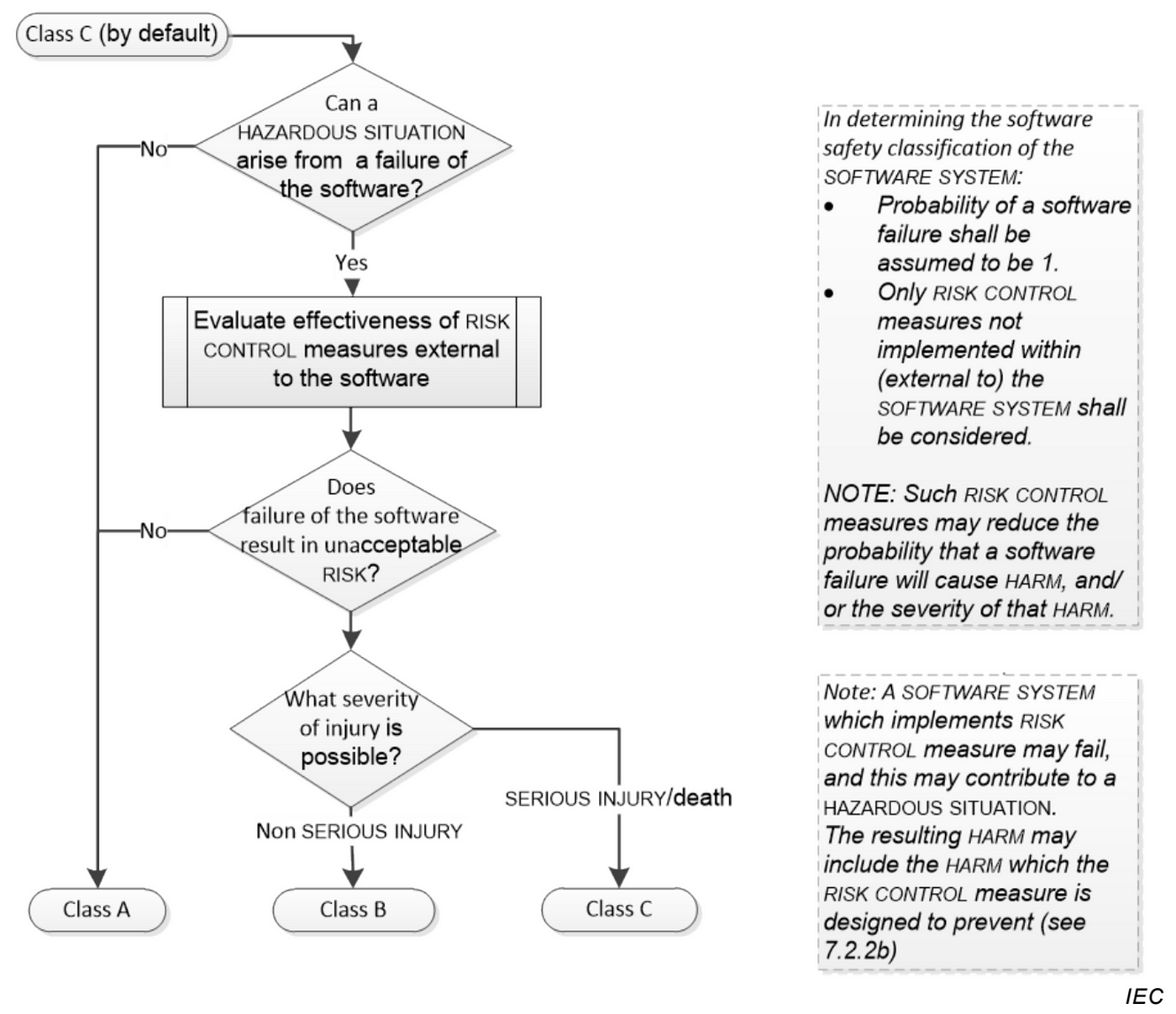
Implementing IEC 62304 for Safe and Effective Medical Device Software — PART 1 - Medical Design Briefs
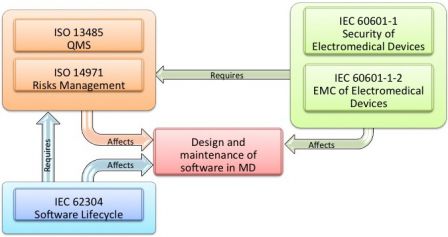
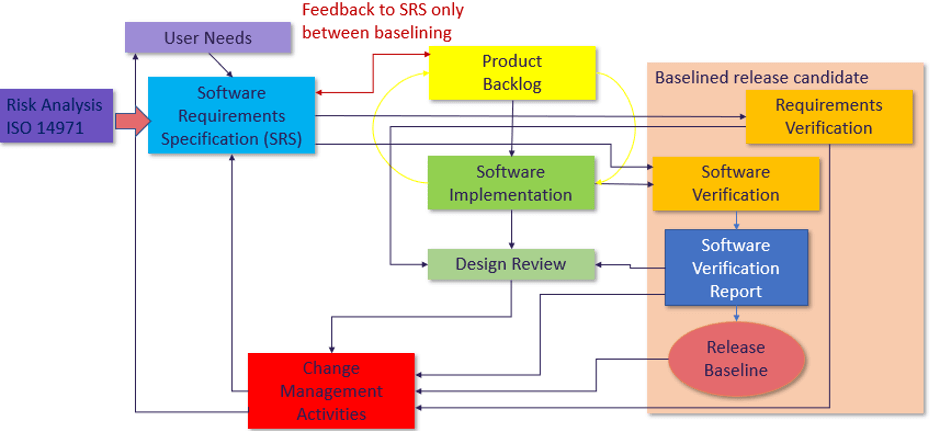
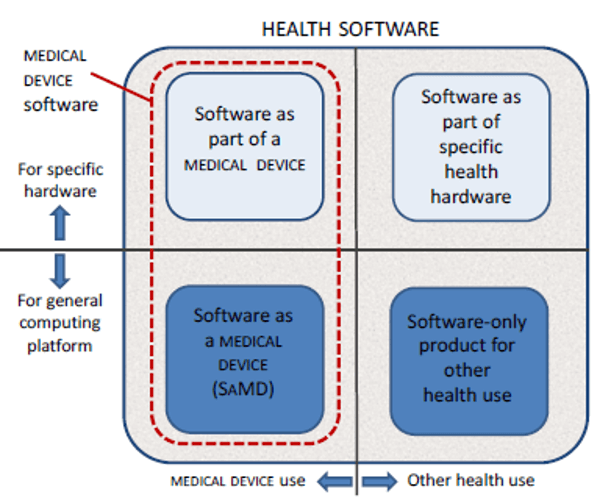
MD and IVD standards: IEC 60601-1 and IEC 61010-1, versus IEC 62304 - Part 1 - Software in Medical Devices, by MD101 Consulting